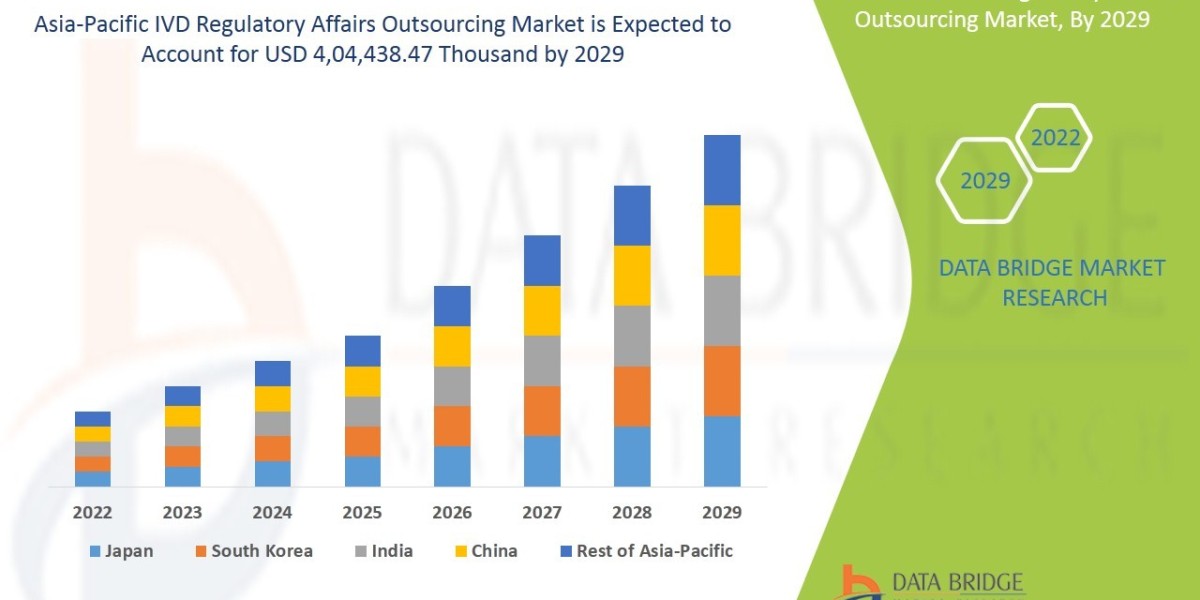
"Executive Summary Asia-Pacific IVD Regulatory Affairs Outsourcing Market :
Data Bridge Market Research analyses that the market is growing with the CAGR of 14.1% in the forecast period of 2022 to 2029 and expected to reach USD 4, 04,438.47 thousand by 2029.
The market insights gained through this Asia-Pacific IVD Regulatory Affairs Outsourcing Market research analysis report facilitates more defined understanding of the market landscape, issues that may interrupt in the future, and ways to position definite brand excellently. With the scrupulous competitor analysis covered in this report, businesses can gauge or analyse the strengths and weak points of the competitors which helps build superior business strategies for their own product. For in depth understanding of market and competitive landscape, this Asia-Pacific IVD Regulatory Affairs Outsourcing Market research report serves a lot of parameters and detailed data about industry.
An effective research methodology used in this Asia-Pacific IVD Regulatory Affairs Outsourcing Market report consists of data models that include market overview and guide, vendor positioning grid, market time line analysis, company positioning grid, company market share analysis, standards of measurement, top to bottom analysis and vendor share analysis. Most relevant, unique, and creditable global market research report has been provided to the valuable customers and clients depending upon their specific business needs. The Asia-Pacific IVD Regulatory Affairs Outsourcing Market report is generated with the systematic gathering and analysis of information about individuals or organizations which is conducted through social and opinion research.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Asia-Pacific IVD Regulatory Affairs Outsourcing Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/asia-pacific-ivd-regulatory-affairs-outsourcing-market
Asia-Pacific IVD Regulatory Affairs Outsourcing Market Overview
**Segments**
- On the basis of service type, the Asia-Pacific IVD regulatory affairs outsourcing market can be segmented into product registration, regulatory writing and publishing, clinical trial applications, regulatory consulting, and others. The product registration segment is expected to showcase significant growth in the market as companies are increasingly outsourcing their regulatory affairs activities to efficiently navigate through the complex regulatory landscape governing in vitro diagnostic products in the Asia-Pacific region.
- Based on the end user, the market can be categorized into IVD manufacturers, CROs, and consultants. The IVD manufacturers segment is anticipated to dominate the market due to the rising demand for regulatory compliance services to meet the stringent regulations set forth by regulatory authorities in the Asia-Pacific region.
- By country, the market can be divided into China, Japan, India, South Korea, Australia, and the rest of Asia-Pacific. China is expected to hold a substantial market share owing to the presence of a large number of IVD manufacturers in the country coupled with the increasing focus on quality and compliance to enter global markets.
**Market Players**
- Some of the key players operating in the Asia-Pacific IVD regulatory affairs outsourcing market include ICON plc, TOXIKON, Covance Inc., PAREXEL International Corporation, Freyr, Accell Clinical Research, Weinberg & Associates, and Pharmanet Development Group. These market players are actively involved in strategic initiatives such as collaborations, partnerships, and acquisitions to enhance their service offerings and expand their geographical presence in the Asia-Pacific region.
The Asia-Pacific IVD regulatory affairs outsourcing market is poised for significant growth driven by the increasing demand for outsourced regulatory services by IVD manufacturers to ensure compliance with the stringent regulatory requirements in the region. The market is witnessing a surge in demand for services such as product registration, regulatory writing, and clinical trial applications to expedite the approval process for in vitro diagnostic products. With a focus on quality, safety, and efficacy, market players are continuously investing in advanced regulatory solutions to support the rapid growth of the IVD sector in the Asia-Pacific region.
The Asia-Pacific IVD regulatory affairs outsourcing market is experiencing an upward trend driven by the growing complexity of regulatory requirements for in vitro diagnostic products in the region. With the rise in demand for outsourced regulatory services, companies are turning to specialized providers for assistance in navigating the intricate regulatory landscape. This trend is particularly evident in the product registration segment, where companies are seeking efficient solutions to comply with regulatory standards while accelerating the market entry of their IVD products. The emphasis on regulatory writing, publishing, and clinical trial applications further underscores the importance of regulatory compliance in the IVD sector in the Asia-Pacific region.
Within the market segments based on end users, IVD manufacturers are emerging as the dominant players in driving the demand for regulatory affairs outsourcing services. The stringent regulations imposed by regulatory authorities in the Asia-Pacific region are compelling manufacturers to seek external expertise to ensure compliance and streamline the approval processes for their products. This trend is fueling the growth of service providers catering to the specific needs of IVD manufacturers, as they aim to enhance their regulatory capabilities and strengthen their market position in the region.
In terms of geographical distribution, China stands out as a key player in the Asia-Pacific IVD regulatory affairs outsourcing market. The country's significant market share can be attributed to its large base of IVD manufacturers and a growing emphasis on quality and compliance standards to access global markets. As China continues to bolster its regulatory framework and regulations for IVD products, the demand for regulatory affairs outsourcing services is expected to surge, presenting lucrative opportunities for market players to expand their presence and capitalize on the evolving regulatory landscape.
Key market players such as ICON plc, TOXIKON, and Covance Inc. are actively engaging in strategic initiatives to strengthen their service offerings and geographical footprint in the Asia-Pacific region. Collaborations, partnerships, and acquisitions are enabling these companies to leverage their expertise and resources to cater to the increasing demand for regulatory affairs outsourcing services in the IVD sector. By aligning their strategies with the evolving market dynamics and regulatory requirements, these players are well-positioned to drive innovation and fuel the growth of the Asia-Pacific IVD regulatory affairs outsourcing market.The Asia-Pacific IVD regulatory affairs outsourcing market is witnessing significant growth driven by the increasing complexity of regulatory requirements in the region. Companies are increasingly turning to specialized service providers to navigate the intricate regulatory landscape governing in vitro diagnostic products. The emphasis on services such as product registration, regulatory writing, and clinical trial applications highlights the crucial role of regulatory compliance in expediting the approval process for IVD products in the Asia-Pacific region.
IVD manufacturers are emerging as the dominant end users fueling the demand for regulatory affairs outsourcing services. With stringent regulations in place, manufacturers are seeking external expertise to ensure compliance and streamline approval processes. This trend is creating opportunities for service providers to tailor their offerings to meet the specific regulatory needs of IVD manufacturers, thereby strengthening their market position in the region.
China stands out as a key player in the Asia-Pacific IVD regulatory affairs outsourcing market due to its large base of IVD manufacturers and increasing focus on quality and compliance standards to access global markets. As China enhances its regulatory framework for IVD products, the demand for regulatory affairs outsourcing services is expected to rise, presenting lucrative opportunities for market players to expand their presence and capitalize on evolving regulatory requirements.
Market players such as ICON plc, TOXIKON, and Covance Inc. are actively pursuing strategic initiatives to enhance their service portfolios and geographical footprint in the Asia-Pacific region. Through collaborations, partnerships, and acquisitions, these companies are leveraging their capabilities to meet the growing demand for regulatory affairs outsourcing services in the IVD sector. By aligning their strategies with market dynamics and regulatory changes, these players are well-positioned to drive innovation and propel the growth of the Asia-Pacific IVD regulatory affairs outsourcing market.
In conclusion, the Asia-Pacific IVD regulatory affairs outsourcing market is poised for continued growth as companies prioritize regulatory compliance to navigate the complex regulatory landscape in the region. With a focus on enhancing regulatory capabilities and ensuring compliance with stringent requirements, market players are well-positioned to capitalize on the expanding demand for outsourced regulatory services in the dynamic IVD sector of the Asia-Pacific region.
The Asia-Pacific IVD Regulatory Affairs Outsourcing Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/asia-pacific-ivd-regulatory-affairs-outsourcing-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
The report can answer the following questions:
- Global major manufacturers' operating situation (sales, revenue, growth rate and gross margin) of Asia-Pacific IVD Regulatory Affairs Outsourcing Market
- Global major countries (United States, Canada, Germany, France, UK, Italy, Russia, Spain, China, Japan, Korea, India, Australia, New Zealand, Southeast Asia, Middle East, Africa, Mexico, Brazil, C. America, Chile, Peru, Colombia) market size (sales, revenue and growth rate) of Asia-Pacific IVD Regulatory Affairs Outsourcing Market
- Different types and applications of Asia-Pacific IVD Regulatory Affairs Outsourcing Market share of each type and application by revenue.
- Global of Asia-Pacific IVD Regulatory Affairs Outsourcing Market size (sales, revenue) forecast by regions and countries from 2022 to 2028 of Asia-Pacific IVD Regulatory Affairs Outsourcing Market
- Upstream raw materials and manufacturing equipment, industry chain analysis of Asia-Pacific IVD Regulatory Affairs Outsourcing Market
- SWOT analysis of Asia-Pacific IVD Regulatory Affairs Outsourcing Market
- New Project Investment Feasibility Analysis of Asia-Pacific IVD Regulatory Affairs Outsourcing Market
Browse More Reports:
Global Industrial Maintenance Services in Operational Improvement and Operational Maintenance Market
Global Digital Calipers with OLED Display Market
Global Hidradenitis Suppurativa Market
Global Mental Illnesses Market
Asia-Pacific Cannabidiol (CBD) Market
Global Operational Analytics Market
Asia-Pacific IoT (Internet of Things) for Public Safety Market
Global Canned Fruits and Vegetable Market
Global Security and Vulnerability Management Market
Global Skincare Supplements Market
Global Asphalt Emulsion Market
Global Seamless Pipes Market
Global Von Hippellindau Syndrome Market
Global Lactic Acid Ester Market
Global Liquid Heat Exchanger System Market
Europe and U.S. Lubricants Market
Global Aluminum Coatings Market
Global Hydrogen Sulfide (H2S) Scavengers Market
Global AI in Education Market
Global Electronic Flight Bag (EFB) Market
U.S. Dental Insurance Market for Individuals – Industry Trends and Forecast to 2031
Global Guillain-Barré Syndrome (GBS) Market
Global Organic Period Care Products Market
Global Aircraft Health Monitoring System Market
Middle East and Africa Chlor-Alkali Market
Global Wi-Fi Semiconductor Chipset Market
Global Baby Care Packaging Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com