"Executive Summary IVD Regulatory Affairs Outsourcing Market :
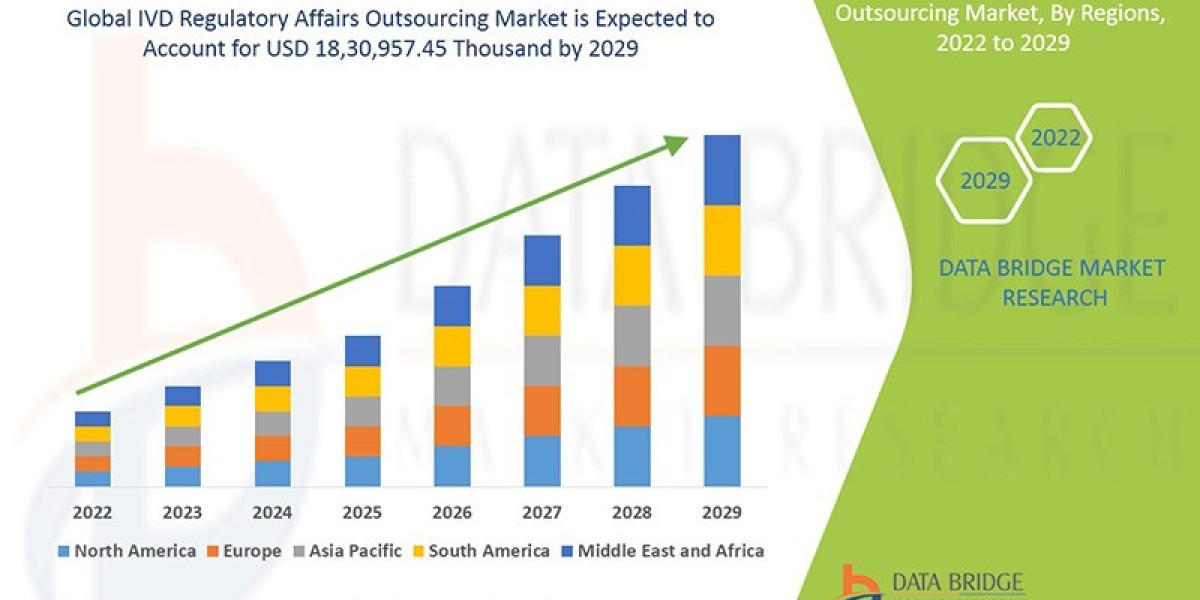
Data Bridge Market Research analyses that the market is growing with the CAGR of 13.3% in the forecast period of 2022 to 2029 and expected to reach USD 18, 30,957.45 thousand by 2029.
The global IVD Regulatory Affairs Outsourcing Market report has been generated with the appropriate expertises that employ established and unswerving tools and techniques such as SWOT analysis and Porter's Five Forces analysis to conduct the research study. Several company profiles included in this IVD Regulatory Affairs Outsourcing Market report can be pretty useful for making any decision associated with revenue, import, export and consumption. This report studies and evaluates facts and figures about the market segmentation very watchfully and represents it in the form of graphs for the better understanding of end user. This market report endows with CAGR value fluctuations during the forecast period of 2018-2025 for the market.
This international IVD Regulatory Affairs Outsourcing Market research report takes into account key product developments and tracks recent acquisitions, mergers and research in the industry by the top market players. According to this business report, the key market players are making moves like product launches, joint ventures, developments, mergers and acquisitions which has influence on the market and Industry as a whole and also affecting the sales, import, export, revenue and CAGR values. This IVD Regulatory Affairs Outsourcing Market report provides the relevant information about specific niche and saves a lot of time that is otherwise taken for decision making.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive IVD Regulatory Affairs Outsourcing Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/global-ivd-regulatory-affairs-outsourcing-market
IVD Regulatory Affairs Outsourcing Market Overview
**Segments**
- **By Product Type**: The market can be segmented into Instruments, Reagents, Services.
- **By Application**: Segmentation can be done into Infectious Diseases, Diabetes, Oncology,Cardiology, Nephrology,Autoimmune Diseases,Drug Testing,Others
- **By End User**: The market can be divided into Hospitals, Laboratories, Academics, Point Of Care Testing,Others
- **By Service Type**: The market can be categorized into Product Registration, Clinical Trial Application, Regulatory Writing And Publishing, Legal Representation, Others
- **By Geography**: This segment can further be subdivided into North America, Europe, Asia-Pacific, South America, Middle East and Africa
**Market Players**
- **Freyr Solutions**
- **Parexel International Corporation**
- **IQVIA**
- **Oriel STAT A Matrix**
- **Registrar Corp**
- **Underwood Consulting Group, LLC**
- **The Weinberg Group**
- **ANDERSON BRECON**
- **RAQAM Consultancy**
- **MET Technologes, Inc.**
The Global IVD Regulatory Affairs Outsourcing Market is experiencing significant growth due to the rising demand for advanced diagnostic solutions, increasing prevalence of chronic and infectious diseases, and the need for efficient regulatory compliance in the healthcare industry. The market segmentation based on product type, application, end user, service type, and geography allows for a comprehensive understanding of the various factors influencing market growth. The increasing focus on precision medicine and personalized healthcare is also a key driver for the growth of the market, as regulatory affairs outsourcing plays a crucial role in ensuring timely market approval and compliance with stringent regulations. The Asia-Pacific region is expected to witness rapid growth in the market, driven by increasing investments in healthcare infrastructure and a growing demand for innovative diagnostic solutions.
Market players such as Freyr Solutions, Parexel International Corporation, and IQVIA are leading the way in providing regulatory affairs outsourcing services to the IVD industry. These companies offer a range of services including product registration, clinical trial application, regulatory writing, and legal representation to help IVD companies navigate complex regulatory landscapes and ensure timely market access for their products. Collaboration with regulatory consulting firms has become a common strategy for market players to enhance their service offerings and expand their market presence. With the increasing focus on regulatory compliance and quality assurance in the healthcare sector, the demand for IVD regulatory affairs outsourcing services is expected to continue to grow in the coming years.
The Global IVD Regulatory Affairs Outsourcing Market is poised for significant expansion driven by several key factors. One emerging trend is the increasing emphasis on digital health solutions and telemedicine, which is reshaping the landscape of the healthcare industry. As technology continues to advance, there is a growing demand for regulatory affairs outsourcing services to help navigate the complex regulatory requirements and ensure compliance with evolving standards. Market players are increasingly focusing on developing innovative approaches to address the unique challenges in the IVD sector, such as personalized medicine and the integration of artificial intelligence in diagnostics.
Furthermore, the COVID-19 pandemic has highlighted the importance of rapid and accurate diagnostic testing, leading to a surge in demand for IVD products and services. This has underscored the critical role of regulatory affairs outsourcing in facilitating timely approvals and ensuring the quality and safety of diagnostic tests. Market players are leveraging this momentum to enhance their service offerings and expand their global footprint, particularly in regions experiencing rapid healthcare infrastructure development and increasing investments in healthcare technology.
Another significant driver of market growth is the increasing adoption of point-of-care testing and decentralized healthcare delivery models. This trend is driving the need for regulatory support services that can streamline market access for IVD products and accelerate time-to-market. Market players are responding to this demand by offering tailored solutions that address the unique regulatory challenges associated with point-of-care testing and remote diagnostics.
Moreover, the growing sophistication of IVD technologies, such as molecular diagnostics and next-generation sequencing, is spurring the need for specialized regulatory expertise. Market players are investing in R&D to develop innovative solutions that meet the evolving regulatory requirements and quality standards in the IVD industry. Collaborations and partnerships between regulatory consulting firms and IVD companies are becoming increasingly common as market players seek to leverage combined expertise and resources to navigate the regulatory landscape effectively.
In conclusion, the Global IVD Regulatory Affairs Outsourcing Market is set for robust growth driven by technological advancements, changing regulatory requirements, and the increasing demand for rapid and accurate diagnostic solutions. Market players are well-positioned to capitalize on these opportunities by offering tailored services that address the unique needs of the IVD industry and support the development of innovative diagnostic products. The evolving regulatory landscape and the increasing focus on quality and compliance will continue to drive the demand for regulatory affairs outsourcing services in the IVD sector, positioning the market for sustained expansion in the years to come.The Global IVD Regulatory Affairs Outsourcing Market is witnessing notable growth driven by various factors influencing the healthcare industry. With the rising demand for advanced diagnostic solutions and the prevalence of chronic and infectious diseases, there is a growing need for efficient regulatory compliance, which is where regulatory affairs outsourcing services play a crucial role. The segmentation of the market based on product type, application, end user, service type, and geography provides a comprehensive understanding of the market dynamics. Moreover, the emphasis on precision medicine and personalized healthcare is contributing to the market growth as companies seek timely market approval and adherence to regulatory standards.
Market players such as Freyr Solutions, Parexel International Corporation, and IQVIA are at the forefront of offering regulatory affairs outsourcing services to the IVD industry. These companies provide a range of services including product registration, clinical trial application, and regulatory writing to facilitate market access for IVD products. Collaborations with regulatory consulting firms are becoming increasingly common as companies aim to enhance their service offerings and expand their market presence. The Asia-Pacific region is expected to experience significant growth in the market due to increased investments in healthcare infrastructure and the demand for innovative diagnostic solutions.
Additionally, the COVID-19 pandemic has underscored the critical importance of rapid and accurate diagnostic testing, leading to a surge in demand for IVD products and services. Market players are leveraging this momentum to strengthen their service offerings and cater to the evolving needs of the healthcare industry. The increasing adoption of point-of-care testing and decentralized healthcare models is driving the demand for regulatory support services to streamline market access for IVD products and accelerate time-to-market. This trend is prompting market players to develop tailored solutions that address the unique regulatory challenges associated with point-of-care testing and remote diagnostics.
Furthermore, the growing sophistication of IVD technologies such as molecular diagnostics is spurring the need for specialized regulatory expertise. Market players are investing in research and development to meet the evolving regulatory requirements and quality standards in the IVD industry. Collaborations between regulatory consulting firms and IVD companies are becoming more prevalent as companies seek to navigate the regulatory landscape effectively and ensure compliance with changing standards. Overall, the Global IVD Regulatory Affairs Outsourcing Market is poised for substantial growth driven by technological advancements, changing regulatory landscapes, and the increasing demand for innovative diagnostic solutions to meet the evolving needs of the healthcare industry.
The IVD Regulatory Affairs Outsourcing Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/global-ivd-regulatory-affairs-outsourcing-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Core Objective of IVD Regulatory Affairs Outsourcing Market:
Every firm in the IVD Regulatory Affairs Outsourcing Market has objectives but this market research report focus on the crucial objectives, so you can analysis about competition, future market, new products, and informative data that can raise your sales volume exponentially.Size of the IVD Regulatory Affairs Outsourcing Market and growth rate factors.
- Important changes in the future IVD Regulatory Affairs Outsourcing Market.
- Top worldwide competitors of the IVD Regulatory Affairs Outsourcing Market.
- Scope and product outlook of IVD Regulatory Affairs Outsourcing Market.
- Developing regions with potential growth in the future.
- Tough Challenges and risk faced in IVD Regulatory Affairs Outsourcing Market.
Global IVD Regulatory Affairs Outsourcing Market top manufacturers profile and sales statistics.
Browse More Reports:
Global Cinnamon Extract Market
Global Corn starch Market
Asia-Pacific Plant-Based Beverages Market
Global Thrombocythemia Market
Asia-Pacific Marine Ingredients Market
Global Pharmacloud Market
North America Image-Guided Surgery Equipment and Navigation-Assisted Surgical Equipment Market
Global Industrial Diaphragm Valve Market
Global Car Mat Market
Global Laptop Backpack Market
Asia-Pacific Electronic Medical Records (EMR) Market
Global Intestine Cancer Therapeutics Market
Global Helideck Monitoring System Market
Global Canoe and Kayak Market
Australia Surface Disinfectant Wipes Market
Global Coil Coatings Market
Global Brewer’s Yeast Market
Global Doppler Ultrasound Systems Market
Middle East and Africa Image-Guided Surgery Equipment and Navigation-Assisted Surgical Equipment Market
Asia-Pacific Chlor-Alkali Market
Europe Depth of Anesthesia Monitoring Market
Global Bruton's Tyrosine Kinase (BKT) Market
Global Medical Tapes and Bandages Market
Asia-Pacific Liquid Chromatography Devices Market
Global Depth of Anesthesia Monitoring Market
Global Cryochambers Market
Europe Industrial Maintenance Services in Operational Improvement and Operational Maintenance Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com